BCN, 18 May 2021.- Scientists led by Dr. Salvador Aznar-Benitah, head of the Stem Cells and Cancer laboratory at IRB Barcelona, have described the alterations that occur during mammary gland formation when heterochromatin (the part of DNA that does not actively produce proteins) is poorly regulated. The results, which have been published in the journal Cell Stem Cell, indicate that incorrect DNA packaging makes retrotransposons (a type of transposable element originated in ancestral fragments of viruses integrated into the cell genome) more accessible. Domenica Marchese and Holger Heyn from the Single Cell Genomics Team at the CNAG-CRG have participated in the study.
As these fragments are more accessible, they can be “read” and copied by the cellular machinery. The cell reacts to the presence of these viral fragments as if it was undergoing infection and it triggers an immune response through a cellular program called pyroptosis. As a result, the interactions between different cells are altered within the breast, leading to complete failure to perform its primary function of milk secretion.
"In many types of cancer, such as triple-negative breast cancer, we observe that there are regions of the genome that are unexpectedly unfolded and we need to understand the implications. The inflammatory response and the alterations that arise could play a key role in the ability of these cells to colonise other organs, causing what is known as metastasis," explains Salvador Aznar-Benitah, ICREA researcher at IRB Barcelona.
The importance of DNA folding
The main function of the DNA in our cells is to coordinate the production of proteins responsible for executing cellular functions. However, some parts of the genome are tightly condensed and thus are not used for this purpose. This study and others published by the scientific community report that proper regulation of chromatin condensation is also key for the correct development of tissues.
The non-coding DNA of animals contains transposable elements, which are ancestral viruses that integrated into the genome a long time ago. If these elements are “read” and copied by the cellular machinery, it is detrimental for the cell. Therefore, the cell has evolved mechanisms to protect itself. One of the mechanisms that prevents retroviral copying in healthy cells is chromatin compaction. In their recent work, Salvador Aznar-Benitah and colleagues demonstrated that disrupting this compaction and releasing these ancestral viruses is not only detrimental to the individual cell but has repercussions on the function of the whole tissue (and maybe even the organism).
“We used single-cell technologies and it was fascinating to see on cellular level how the altered genome compaction affected the development of complex breast tissue. Analyzing one cell at a time revealed stark differences in the composition of building blocks that form tissue architecture, but also identified cell states that are never seen in healthy mice", says Holger Heyn, CNAG-CRG's Single Cell Genomics Team leader.
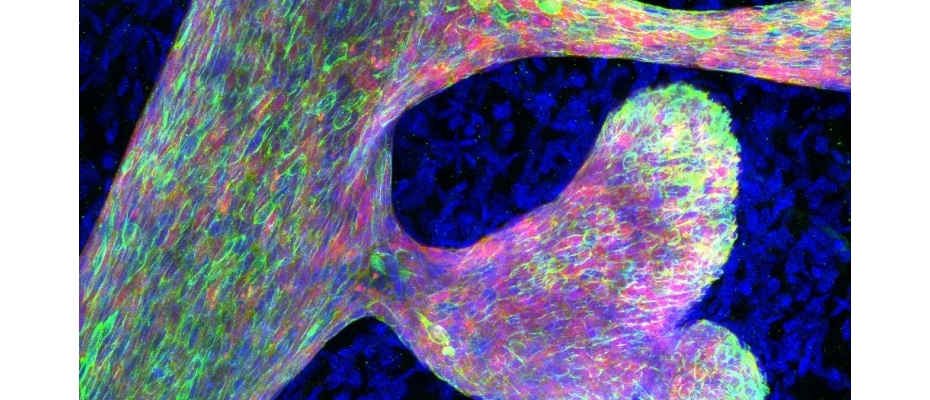
Image Caption: Immunofluorescence staining for mammary epithelium (red), highlighting the luminal lineage (green), showing aberrant mammary branching upon perturbed chromatin accessibility (IRB Barcelona)
Work of reference: Repression of endogenous retroviruses is required for mammary gland development
Source: IRB Barcelona










